
Creek Road General Practice now has stock of the most recent adult COVID-19 vaccination available: Bivalent Pfizer Original/Omicron BA.4/5 vaccine.
As winter 2023 approaches below is an update regarding COVID-19 vaccination recommendations:
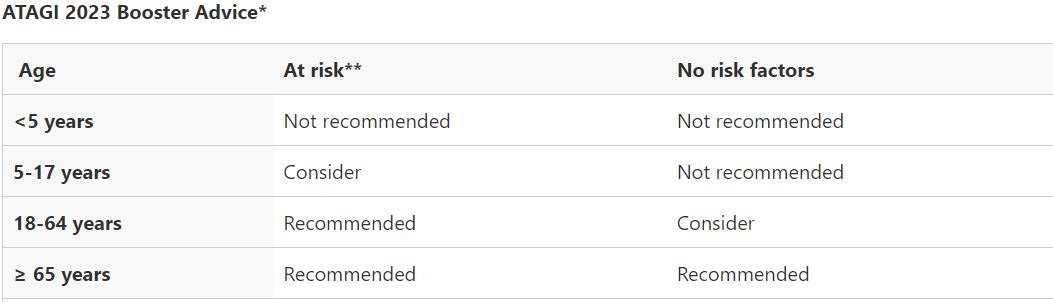
An excerpt from the recommendations which answers most questions is below. The full ATAGI recommendations are available here. Please note these are general recommendations, if you have questions specific to your situation please discuss with your treating doctor.
- ATAGI recommends a 2023 COVID-19 vaccine booster dose for adults in the following groups, if their last COVID-19 vaccine dose or confirmed infection (whichever is the most recent) was 6 months ago or longer, and regardless of the number of prior doses received:
- All adults aged 65 years and over
- Adults aged 18-64 years who have medical comorbidities that increase their risk of severe COVID-19, or disability with significant or complex health needs.
- ATAGI advises the following groups should consider a 2023 booster dose if their last COVID-19 vaccine dose or confirmed infection (whichever is the most recent) was 6 months ago or longer, and regardless of the number of prior doses received, based on an individual risk benefit assessment with their immunisation provider.
- All Adults aged 18-64 years without risk factors for severe COVID-19
- Children and adolescents aged 5-17 years who have medical comorbidities that increase their risk of severe COVID-19, or disability with significant or complex health needs.
- ATAGI advises that a booster dose is not recommended at this time for children and adolescents aged under the age of 18 who do not have any risk factors for severe COVID-19.
- Regarding vaccine choice, all currently available COVID-19 vaccines are anticipated to provide benefit as a booster dose, however bivalent mRNA booster vaccines are preferred over other vaccines. These include: Pfizer Original/Omicron BA.4/5, as well as Pfizer Original/Omicron BA.1 or Moderna Original/Omicron BA.1. Moderna Original/Omicron BA.4/5 is currently under evaluation by the Therapeutic Goods Administration.
- COVID-19 vaccine can be co-administered with influenza and other vaccines.
- Administration of a 2023 COVID-19 booster dose should aim to occur prior to June 2023 and at a time of 6 months or greater following the most recent COVID-19 vaccine dose or confirmed infection.
