With Autumn upon us, the Influenza Vaccinations for the 2021 season are almost here.
Creek Road General Practice will have stock of both Private Vaccinations and Government Vaccinations in early to mid April. With this in mind, this blog post will cover what you need to know regarding Flu Vaccination in 2021.
When should I have the Flu Vaccination?
As per current Queensland Health recommendations, April is the earliest you should have the flu vaccination, unless there is a specific exposure you are concerned about, and discuss with your doctor. Further details are available from Queensland Health, but here is a summary:
The timing of vaccination should aim to achieve the highest level of protection during the peak of the influenza season. Flu season in Queensland is typically from June to September, with the peak usually in August.
Vaccinating from April provides protection before the peak season takes place. While protection is generally expected to last for the whole season, the best protection against influenza occurs within the first 3 to 4 months following vaccination.
Which Flu Vaccination should I get?
All vaccinations available in Australia this year are quadrivalent, meaning they all cover 4 strains of the flu virus. The one vaccination which is a bit different is Fluad Quad, which is specifically targeted at over 65s. As per Queensland Health we strongly recommend that every patient over 65 visit their GP to have Fluad Quad, rather than receiving a standard flu vaccination:
It is an adjuvanted (or enhanced) vaccine which is a standard dose flu vaccine with an added adjuvant to help create a stronger immune response to the vaccination. The 2021 vaccine contains the same components as the vaccine provided for other age groups but has the benefit of inducing a greater immune response in older people.
The enhanced quadrivalent influenza vaccine is the best form of protection against flu for older Queenslanders for the following reasons:
-
Older people do not respond as well to standard influenza vaccine as the immune system response decreases with age.
-
The enhanced vaccine is designed specifically to increase the immune system’s response to the vaccine, especially against the influenza A/H3N2 strain which is more common and severe in people aged 65 years and older.
What about COVID-19 Vaccination and Flu Vaccination?
This year there are additional considerations with the arrival of the COVID-19 vaccination, and the recommendation from Australian Technical Advisory Group on Immunisation (ATAGI) that there should be at least a 2 week gap between the COVID-19 vaccination and flu vaccination (not due to any risk associated with having them together, but to better track any possible vaccination side effects).
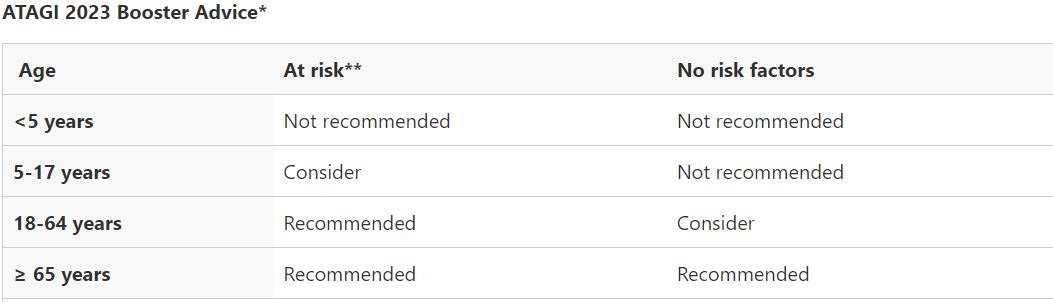
Am I Eligible for Government Funded (Free) Flu Vaccination?
Per guidelines from NCIRS, Annual influenza vaccination is both recommended and funded under the National Immunisation Program (NIP) for people aged ≥6 months who are at increased risk of severe influenza, including:
– all Aboriginal and/or Torres Strait Islander people aged ≥6 months
– all children aged 6 months to <5 years
– all adults aged ≥65 years
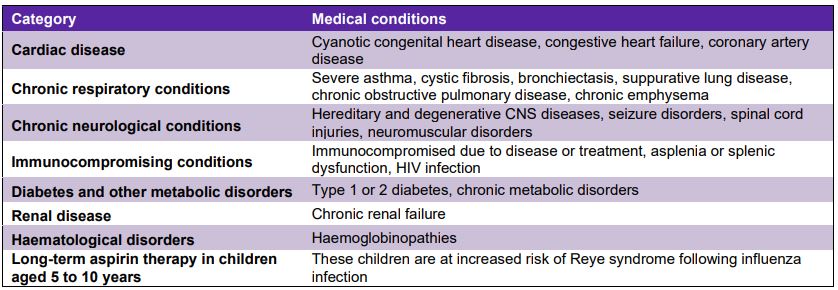
– people with specified medical conditions (refer to List below)
– pregnant women (during any stage of pregnancy).
- Cardiac disease – Cyanotic congenital heart disease, Congestive heart failure, Coronary artery disease
- Chronic respiratory conditions – Severe asthma (for which frequent medical consultations or the use of multiple medications is required), Cystic fibrosis, Bronchiectasis, Suppurative lung disease, Chronic obstructive pulmonary disease (COPD), Chronic emphysema
- Chronic neurological conditions – Hereditary and degenerative CNS diseases (including multiple sclerosis), Seizure disorders, Spinal cord injuries, Neuromuscular disorders
- Immunocompromising conditions – Immunocompromised due to disease or treatment (e.g. malignancy, transplantation and/or chronic steroid use), Asplenia or splenic dysfunction, HIV infection
- Diabetes and other metabolic disorders – Type 1 diabetes ,Type 2 diabetes, Chronic metabolic disorders
- Renal disease – Chronic renal failure
- Haematological disorders – Haemoglobinopathies
- Long-term aspirin therapy in children aged 6 months to 10 years
As always, if you have specific questions regarding the Flu vaccination and your own health, book in to discuss further with your GP.